Kelvin
| from Kelvin | to Kelvin | |
|---|---|---|
| Celsius | [°C] = [K] − 273.15 | [K] = [°C] + 273.15 |
| Fahrenheit | [°F] = [K] × 9⁄5 − 459.67 | [K] = ([°F] + 459.67) × 5⁄9 |
| Rankine | [°R] = [K] × 9⁄5 | [K] = [°R] × 5⁄9 |
| For temperature intervals rather than specific temperatures, 1 K = 1 °C = 1.8 °F = 1.8 °R Comparisons among various temperature scales |
||
The kelvin (symbol: K) is a unit increment of temperature and is one of the seven SI base units. The Kelvin scale is a thermodynamic (absolute) temperature scale referenced to absolute zero, the absence of all thermal energy. So by definition, the temperature of a substance at absolute zero is zero kelvin (0 K). The secondary reference point on the Kelvin scale is the triple point of water (0.01 degrees Celsius). The Kelvin scale is the difference between these two reference points, with the kelvin defined as one 273.16th of this scale. The Kelvin scale and the kelvin are named after the Belfast-born physicist and engineer William Thomson, 1st Baron Kelvin (1824–1907), who wrote of the need for an "absolute thermometric scale". Unlike the degree Fahrenheit and degree Celsius, the kelvin is not referred to as a "degree", nor is it typeset with a degree symbol; that is, it is written K and not °K. The kelvin and the degree Celsius are often used together, as they have the same interval, and 0 kelvin is −273.15 degrees Celsius.
Contents |
History
- 1848
- Lord Kelvin (William Thomson), wrote in his paper, On an Absolute Thermometric Scale, of the need for a scale whereby "infinite cold" (absolute zero) was the scale’s null point, and which used the degree Celsius for its unit increment. Thomson calculated that absolute zero was equivalent to −273 °C on the air thermometers of the time.[1] This absolute scale is known today as the Kelvin thermodynamic temperature scale. Thomson’s value of "−273" was the reciprocal of 0.00366 - the accepted expansion coefficient of gas per degree Celsius relative to the ice point, giving a remarkable consistency to the currently accepted value.
- 1954
- Resolution 3 of the 10th CGPM gave the Kelvin scale its modern definition by designating the triple point of water as its second defining point and assigned its temperature to exactly 273.16 kelvin.[2]
- 1967/1968
- Resolution 3 of the 13th CGPM renamed the unit increment of thermodynamic temperature "kelvin", symbol K, replacing "degree absolute", symbol °K.[3] Furthermore, feeling it useful to more explicitly define the magnitude of the unit increment, the 13th CGPM also held in Resolution 4 that "The kelvin, unit of thermodynamic temperature, is equal to the fraction 1/273.16 of the thermodynamic temperature of the triple point of water."[4]
- 2005
- The Comité International des Poids et Mesures (CIPM), a committee of the CGPM, affirmed that for the purposes of delineating the temperature of the triple point of water, the definition of the Kelvin thermodynamic temperature scale would refer to water having an isotopic composition specified as VSMOW.[5]
Usage conventions
When reference is made to the unit kelvin (either a specific temperature or a temperature interval), kelvin is always spelled with a lowercase k unless it is the first word in a sentence.[6] When reference is made to the "Kelvin scale", the word "kelvin"—which is normally a noun—functions adjectivally to modify the noun "scale" and is capitalized.
Until the 13th General Conference on Weights and Measures (CGPM) in 1967–1968, the unit kelvin was called a "degree", the same as with the other temperature scales at the time. It was distinguished from the other scales with either the adjective suffix "Kelvin" ("degree Kelvin") or with "absolute" ("degree absolute") and its symbol was °K. The latter (degree absolute), which was the unit’s official name from 1948 until 1954, was rather ambiguous since it could also be interpreted as referring to the Rankine scale. Before the 13th CGPM, the plural form was "degrees absolute". The 13th CGPM changed the name to simply "kelvin" (symbol K).[7] The omission of "degree" indicates that it is not relative to an arbitrary reference point like the Celsius and Fahrenheit scales, but rather an absolute unit of measure which can be manipulated algebraically (e.g., multiplied by two to indicate twice the amount of "mean energy" available among elementary degrees of freedom of the system).
This SI unit is named after William Thomson, 1st Baron Kelvin. As with every SI unit whose name is derived from the proper name of a person, the first letter of its symbol is uppercase (K). When an SI unit is spelled out in English, it should always begin with a lowercase letter (kelvin), except where any word would be capitalized, such as at the beginning of a sentence or in capitalized material such as a title. Note that "degree Celsius" conforms to this rule because the "d" is lowercase.—Based on The International System of Units, section 5.2.
The kelvin symbol is always a roman, non-italic capital K. In the SI naming convention, all symbols named after a person are capitalized; in the case of the kelvin, capitalizing also distinguishes the symbol from the SI prefix "kilo", which has the lowercase k as its symbol. The admonition against italicizing the symbol K applies to all SI unit symbols; only symbols for variables and constants (e.g., P = pressure, and c = 299,792,458 m/s) are italicized in scientific and engineering papers. As with most other SI unit symbols (angle symbols, e.g. 45° 3′ 4″, are the exception) there is a space between the numeric value and the kelvin symbol (e.g. "99.987 K").[8][9]
Unicode provides a compatibility character for the kelvin at U+212A (decimal 8490), for compatibility with CJK encodings that provide such a character (as such, in most fonts the width is the same as for fullwidth characters).
Use in conjunction with Celsius
In science and in engineering, the Celsius scale and the kelvin are often used simultaneously in the same article (e.g., "...its measured value was 0.01028 °C with an uncertainty of 60 µK..."). This practice is permissible because the degree Celsius is a special name for the kelvin for use in expressing Celsius temperatures and the magnitude of the degree Celsius is exactly equal to that of the kelvin.[10] Notwithstanding that the official endorsement provided by Resolution 3 of the 13th CGPM states, "a temperature interval may also be expressed in degrees Celsius," the practice of simultaneously using both "°C" and "K" remains widespread throughout the scientific world as the use of SI prefixed forms of the degree Celsius (such as "µ°C" or "microdegrees Celsius") to express a temperature interval has not been widely adopted. A helpful way to think of the kelvin system is thinking that nothing can be colder than 0 kelvin (-273.15 degrees Celsius).[3]
Proposed redefinition
In 2005 the CIPM embarked on a program to redefine, amongst others, the Kelvin using a more rigorous basis than was in use. The current (2010) definition is unsatisfactory for temperatures below 20 K and above 1300 K.[11] It is anticipated that the program will be completed in time for its adoption by the CGPM at its 2011 meeting. The committee proposes defining the kelvin as the temperature scale for which Boltzmann's Constant is 1.3806505×10−23 J/K exactly.
From a scientific point of view, this will link temperature to the rest of SI and result in a stable definition that is independent of any particular substance. From a practical point of view the redefinition will pass unnoticed; water will still freeze at 0 °C (273.15 K).[12]
Practical uses
Color temperature
The kelvin is often used in the measure of the color temperature of light sources. Color temperature is based upon the principle that a black body radiator emits light whose color depends on the temperature of the radiator. Black bodies with temperatures below about 4000 K appear reddish whereas those above about 7500 K appear bluish. Color temperature is important in the fields of image projection and photography where a color temperature of approximately 5600 K is required to match "daylight" film emulsions. In astronomy, the stellar classification of stars and their place on the Hertzsprung-Russell diagram are based, in part, upon their surface temperature, known as effective temperature. The photosphere of the Sun, for instance, has an effective temperature of 5778 K.
Kelvin as a measure of noise
In electronics, the kelvin is used as an indicator of how noisy a circuit is in relation to an ultimate noise floor, i.e. the noise temperature. The so-called Johnson–Nyquist noise of discrete resistors and capacitors is a type of thermal noise derived from the Boltzmann constant and can be used to determine the noise temperature of a circuit using the Friis formulas for noise.
Temperature conversion between units

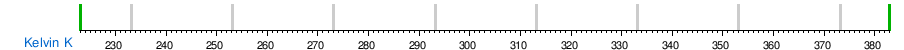
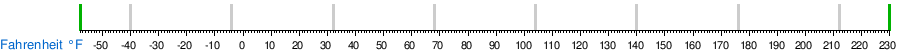

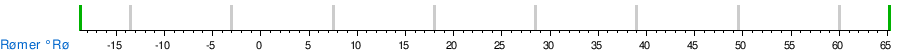



See also
- Comparison of temperature scales
- International Temperature Scale of 1990
- Negative temperature
- Rankine scale
- Thermodynamic temperature
- Triple point
References
- ↑ Thomson, William (October 1848). "On an Absolute Thermometric Scale". Philosophical Magazine. http://zapatopi.net/kelvin/papers/on_an_absolute_thermometric_scale.html. Retrieved 2008-02-06.
- ↑ "Resolution 3: Definition of the thermodynamic temperature scale". Resolutions of the 10th CGPM. Bureau International des Poids et Mesures. 1954. http://www.bipm.fr/en/CGPM/db/10/3/. Retrieved 2008-02-06.
- ↑ 3.0 3.1 "Resolution 3: SI unit of thermodynamic temperature (kelvin)". Resolutions of the 13th CGPM. Bureau International des Poids et Mesures. 1967. http://www.bipm.fr/en/CGPM/db/13/3/. Retrieved 2008-02-06.
- ↑ "Resolution 4: Definition of the SI unit of thermodynamic temperature (kelvin)". Resolutions of the 13th CGPM. Bureau International des Poids et Mesures. 1967. http://www.bipm.fr/en/CGPM/db/13/4/. Retrieved 2008-02-06.
- ↑ "Unit of thermodynamic temperature (kelvin)". SI Brochure, 8th edition. Bureau International des Poids et Mesures. 1967. pp. Section 2.1.1.5. http://www1.bipm.org/en/si/si_brochure/chapter2/2-1/2-1-1/kelvin.html. Retrieved 2008-02-06.
- ↑ BIPM: SI brochure, Section 5.2
- ↑ Barry N. Taylor (2008) (.PDF). Guide for the Use of the International System of Units (SI). Special Publication 811. National Institute of Standards and Technology. http://physics.nist.gov/Document/sp811.pdf. Retrieved 2009-06-24.
- ↑ "SI Unit rules and style conventions". National Institute of Standards and Technology. September 2004. http://physics.nist.gov/cuu/Units/checklist.html. Retrieved 2008-02-06.
- ↑ "Rules and style conventions for expressing values of quantities". SI Brochure, 8th edition. Bureau International des Poids et Mesures. 1967. pp. Section 5.3.3. http://www.bipm.org/en/si/si_brochure/chapter5/5-3-2.html#5-3-3. Retrieved 2008-02-06.
- ↑ "Units with special names and symbols; units that incorporate special names and symbols". SI Brochure, 8th edition. Bureau International des Poids et Mesures. 2006. pp. Section 2.2.2, Table 3. http://www.bipm.org/en/si/si_brochure/chapter2/2-2/table3.html. Retrieved 2008-02-06.
- ↑ J. Fischer1 et al. "Report to the CIPM on the implications of changing the definition of the base unit Kelvin". International Committee for Weights and Measures (CIPM). http://www.bipm.org/wg/CCT/TG-SI/Allowed/Documents/Report_to_CIPM_2.pdf. Retrieved 2010-02-23.
- ↑ "Updating the definition of the kelvin". International Bureau for Weights and Measures (BIPM). http://www.bipm.org/wg/CCT/TG-SI/Allowed/Documents/Updating_the_definition_of_the_kelvin2.pdf. Retrieved 2010-02-23.
External links
- Bureau International des Poids et Mesures (2006) (.PDF). The International System of Units (SI) Brochure. 8th Edition. International Committee for Weights and Measures. http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf. Retrieved 2008-02-06.
- Convert Kelvin to Fahrenheit and Celsius Instantly, Kelvin to Fahrenheit Conversion
|
||||||||
|
||||||||||||||||||||
